Abstract
Introduction The treatment of chronic myeloid leukemia (CML) was revolutionized in 2001 with the introduction of the BCR-ABL tyrosine kinase inhibitor (TKI), imatinib. In 2006, dasatinib was approved, shortly followed by nilotinib in 2007 for the treatment of CML patients (pts) who developed resistance or intolerance to imatinib. In the following years, dasatinib and nilotinib were approved for newly diagnosed CML. Two other TKIs, bosutinib and ponatinib were approved in 2012. Dasatinib, nilotinib and bosutinib are effective against all imatinib resistant mutations except the gatekeeper mutation, T315I. Ponatinib approval provided a TKI option for pts with T315I mutation. In this study we examine how survival has improved in CML pts over time with the availability of multiple TKI options and identify traditionally disadvantaged socioeconomic and demographic groups which continue to have disparate survival outcomes.
Methods The National Cancer Database was used to identify CML pts by histology code 9863 & 9875, who were diagnosed between 2004-2019. Demographic and treatment characteristics were compared by era of TKI availability. Pts diagnosed from 2004-2005 were considered to be treated in the imatinib era, 2006-2011 as having access to dasatinib and nilotinib, and from 2012 on to have access to bosutinib and ponatinib. Pts who received allogeneic transplant were excluded in survival analysis. Kaplan Meier and Cox regression analysis were used to compare overall survival (OS) for the entire cohort and by year (yr) of diagnosis, with adjustment by age, Charleson-Deyo comorbidity, race, sex, insurance, and quartile of income and education level of the patient's zip code to identify demographic and socioeconomic features associated with poor survival outcomes.
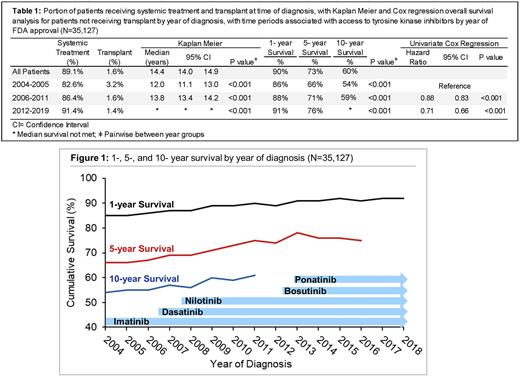
Results Of the 38,763 CML pts identified, 55.5% were male and 81% were white, with a median age of 57 yrs (interquartile range 44-70). The portion of pts who were untreated, either due to comorbidity, death, or contraindication, decreased from 17.4% in the imatinib era to only 8.6% from 2012 on, p<0.001. Though the rate of upfront transplant was low (1.6%), it decreased with the advent of new therapies, from a rate of 3.4% from 2004-2005, to 1.4% from 2012 on, p<0.001. The median OS of CML pts since 2004 is 14.4 years (yrs) (95% confidence interval (CI) 14.0-14.9). For pts diagnosed from 2004-2005 median OS was 12.0 yrs (95% CI 11.1-13.0). This increased to 13.8 yrs (95% CI 13.4-14.2), with a hazard ratio (HR) of 0.88 (95% CI 0.84-0.94) for pts diagnosed 2006-2011 when referenced to the initial time period, p<0.001. Median OS was not reached for the latest time period, with a HR of 0.71 (95% CI 0.66-0.75, p<0.001) referenced to 2004-2006. Survival at 1-, 5- & 10-yrs was improved for each subsequent time period of diagnosis, with a 5-yr OS of 66%, 71%, and 76% for each subsequent diagnosis period, p<0.001. On multivariate cox regression adjusted for yr of diagnosis, features associated with reduced OS included age (HR 1.05 [95% CI 1.05-1.06], p<0.001), increased comorbidity burden (HR 1.34 [95% CI 1.30-1.37], p<0.001), uninsured status (HR 1.95 [95% CI 1.75-2.16], p<0.001), Medicaid (HR 2.2 [95% CI 2.0-2.4], p<0.001), and living in a zip code with median income in the lowest quartile (HR 1.15 [95% CI 1.06-1.25], p<0.001) and lowest education quartile (HR 1.19 [95% CI 1.10-1.29], p<0.001). Features associated with improved survival include female sex (HR 0.80 [0.77-0.84], p<0.001), and subsequent diagnosis year (HR 0.96 [95% CI 0.95-0.96], p<0.001).
Discussion Overall survival for CML pts has significantly improved during the last 18 years, with improvements seen with the availability of each additional TKI. While treatment data is very limited in this dataset, this improvement is likely related to increased availability of multiple TKIs for pts with resistance or intolerance to prior TKI therapies. Additional TKI options, particularly approval of ponatinib for pts with T315I mutation also have a secondary benefit of decreasing the utilization of allogenic transplant, a high-risk procedure in itself. Though survival has improved for all pts, pts from traditionally underserved populations, including those who are underinsured or from low-income or low-education areas, have reduced survival. Continued advances in treatment and efforts to improve access to care for underserved populations is vital in the goal to achieve equitable survival outcomes in CML.
Disclosures
Tantravahi:Karyopharm Therapeutics Inc.,: Research Funding; Karyopharm Therapeutics Inc., Novartis, AbbVie, Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.